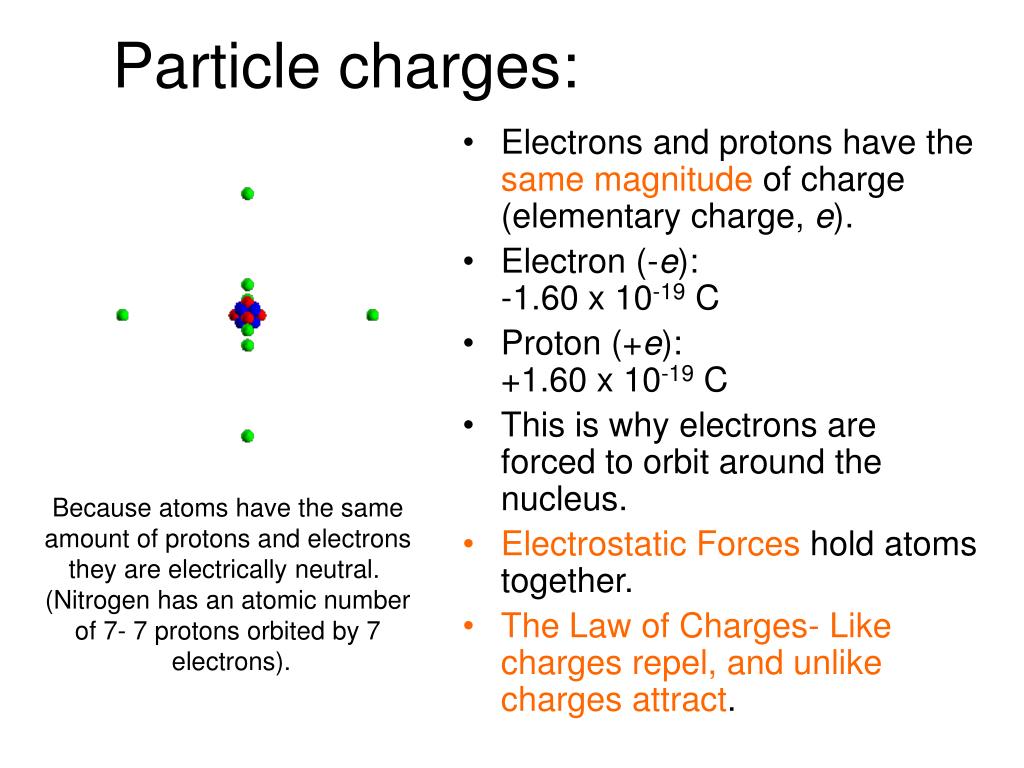
What Are The Electrical Charges Of Subatomic Particles . Discovered by james chadwick in 1932. many fundamental, or subatomic, particles of matter have the property of electric charge. Discovered by ernest rutherford in 1919. For example, electrons have negative charge and. Reside in the nucleus of an atom. Approximately 1 atomic mass unit (amu). A quantum number that determines the electromagnetic interactions of some subatomic particles; know the basics of the experiments involving the discoveries of the three subatomic particles; A quantum number that determines the electromagnetic interactions of some subatomic particles; the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. Memorize relative charge values and amu.
from www.slideserve.com
Discovered by ernest rutherford in 1919. A quantum number that determines the electromagnetic interactions of some subatomic particles; For example, electrons have negative charge and. know the basics of the experiments involving the discoveries of the three subatomic particles; many fundamental, or subatomic, particles of matter have the property of electric charge. A quantum number that determines the electromagnetic interactions of some subatomic particles; Approximately 1 atomic mass unit (amu). Memorize relative charge values and amu. Reside in the nucleus of an atom. Discovered by james chadwick in 1932.
PPT Electricity PowerPoint Presentation, free download ID6751678
What Are The Electrical Charges Of Subatomic Particles Reside in the nucleus of an atom. A quantum number that determines the electromagnetic interactions of some subatomic particles; A quantum number that determines the electromagnetic interactions of some subatomic particles; Discovered by james chadwick in 1932. many fundamental, or subatomic, particles of matter have the property of electric charge. know the basics of the experiments involving the discoveries of the three subatomic particles; For example, electrons have negative charge and. Memorize relative charge values and amu. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. Discovered by ernest rutherford in 1919. Approximately 1 atomic mass unit (amu). Reside in the nucleus of an atom.
From learn.sparkfun.com
What is Electricity? SparkFun Learn What Are The Electrical Charges Of Subatomic Particles Discovered by ernest rutherford in 1919. Approximately 1 atomic mass unit (amu). Discovered by james chadwick in 1932. A quantum number that determines the electromagnetic interactions of some subatomic particles; Reside in the nucleus of an atom. For example, electrons have negative charge and. A quantum number that determines the electromagnetic interactions of some subatomic particles; know the basics. What Are The Electrical Charges Of Subatomic Particles.
From sciencenotes.org
Subatomic Particles What Are The Electrical Charges Of Subatomic Particles many fundamental, or subatomic, particles of matter have the property of electric charge. Discovered by james chadwick in 1932. A quantum number that determines the electromagnetic interactions of some subatomic particles; Reside in the nucleus of an atom. Discovered by ernest rutherford in 1919. Approximately 1 atomic mass unit (amu). know the basics of the experiments involving the. What Are The Electrical Charges Of Subatomic Particles.
From slideplayer.com
ELECTRIC CHARGES AND FORCES. ELECTRIC CHARGE “Charge” is a property of What Are The Electrical Charges Of Subatomic Particles Reside in the nucleus of an atom. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. many fundamental, or subatomic, particles of matter have the property of electric charge. Approximately 1 atomic mass unit (amu). A quantum number that determines the electromagnetic interactions. What Are The Electrical Charges Of Subatomic Particles.
From slidetodoc.com
Subatomic Particles particle symbols Relative electric charge Mass What Are The Electrical Charges Of Subatomic Particles Discovered by ernest rutherford in 1919. many fundamental, or subatomic, particles of matter have the property of electric charge. know the basics of the experiments involving the discoveries of the three subatomic particles; Reside in the nucleus of an atom. A quantum number that determines the electromagnetic interactions of some subatomic particles; A quantum number that determines the. What Are The Electrical Charges Of Subatomic Particles.
From lessonliblehner.z21.web.core.windows.net
How To Explain Using Subatomic Particles What Are The Electrical Charges Of Subatomic Particles many fundamental, or subatomic, particles of matter have the property of electric charge. A quantum number that determines the electromagnetic interactions of some subatomic particles; Memorize relative charge values and amu. A quantum number that determines the electromagnetic interactions of some subatomic particles; the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with. What Are The Electrical Charges Of Subatomic Particles.
From www.coscinecreative.com
How Does Changing Numbers of Subatomic Particles Effect the Atom What Are The Electrical Charges Of Subatomic Particles Reside in the nucleus of an atom. A quantum number that determines the electromagnetic interactions of some subatomic particles; many fundamental, or subatomic, particles of matter have the property of electric charge. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. Memorize relative. What Are The Electrical Charges Of Subatomic Particles.
From www.numerade.com
On the Inside Part l Label the parts of this atom (nucleus, protons What Are The Electrical Charges Of Subatomic Particles A quantum number that determines the electromagnetic interactions of some subatomic particles; know the basics of the experiments involving the discoveries of the three subatomic particles; Reside in the nucleus of an atom. many fundamental, or subatomic, particles of matter have the property of electric charge. the nuclei of all atoms contain subatomic particles called protons close. What Are The Electrical Charges Of Subatomic Particles.
From slideplayer.com
Subatomic Particles Electrical Charge Location Size Protons Positive What Are The Electrical Charges Of Subatomic Particles A quantum number that determines the electromagnetic interactions of some subatomic particles; Discovered by ernest rutherford in 1919. For example, electrons have negative charge and. Discovered by james chadwick in 1932. Reside in the nucleus of an atom. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative. What Are The Electrical Charges Of Subatomic Particles.
From www.vrogue.co
Subatomic Particles You Should Know vrogue.co What Are The Electrical Charges Of Subatomic Particles Reside in the nucleus of an atom. A quantum number that determines the electromagnetic interactions of some subatomic particles; Discovered by ernest rutherford in 1919. many fundamental, or subatomic, particles of matter have the property of electric charge. Approximately 1 atomic mass unit (amu). Discovered by james chadwick in 1932. the nuclei of all atoms contain subatomic particles. What Are The Electrical Charges Of Subatomic Particles.
From www.youtube.com
3.4 Subatomic Particles Mass and Charge YouTube What Are The Electrical Charges Of Subatomic Particles Memorize relative charge values and amu. For example, electrons have negative charge and. Discovered by james chadwick in 1932. Discovered by ernest rutherford in 1919. know the basics of the experiments involving the discoveries of the three subatomic particles; the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and. What Are The Electrical Charges Of Subatomic Particles.
From www.nagwa.com
Question Video Identifying the Relative Charge Values of Subatomic What Are The Electrical Charges Of Subatomic Particles Reside in the nucleus of an atom. Discovered by ernest rutherford in 1919. many fundamental, or subatomic, particles of matter have the property of electric charge. A quantum number that determines the electromagnetic interactions of some subatomic particles; Approximately 1 atomic mass unit (amu). know the basics of the experiments involving the discoveries of the three subatomic particles;. What Are The Electrical Charges Of Subatomic Particles.
From www.askiitians.com
Basic Properties of Electric Charge Study Material for IIT JEE What Are The Electrical Charges Of Subatomic Particles many fundamental, or subatomic, particles of matter have the property of electric charge. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. Approximately 1 atomic mass unit (amu). A quantum number that determines the electromagnetic interactions of some subatomic particles; A quantum number. What Are The Electrical Charges Of Subatomic Particles.
From classfullfauvette.z13.web.core.windows.net
How To Explain Using Subatomic Particles What Are The Electrical Charges Of Subatomic Particles Discovered by james chadwick in 1932. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. Approximately 1 atomic mass unit (amu). Discovered by ernest rutherford in 1919. Reside in the nucleus of an atom. A quantum number that determines the electromagnetic interactions of some. What Are The Electrical Charges Of Subatomic Particles.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science What Are The Electrical Charges Of Subatomic Particles many fundamental, or subatomic, particles of matter have the property of electric charge. For example, electrons have negative charge and. Discovered by james chadwick in 1932. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a relative mass of 1. Memorize relative charge values and amu. Approximately 1. What Are The Electrical Charges Of Subatomic Particles.
From www.slideserve.com
PPT Electricity PowerPoint Presentation, free download ID6751678 What Are The Electrical Charges Of Subatomic Particles Discovered by ernest rutherford in 1919. A quantum number that determines the electromagnetic interactions of some subatomic particles; For example, electrons have negative charge and. know the basics of the experiments involving the discoveries of the three subatomic particles; many fundamental, or subatomic, particles of matter have the property of electric charge. Discovered by james chadwick in 1932.. What Are The Electrical Charges Of Subatomic Particles.
From frecetovbpschematic.z4.web.core.windows.net
Diagram On An Atom What Are The Electrical Charges Of Subatomic Particles Approximately 1 atomic mass unit (amu). know the basics of the experiments involving the discoveries of the three subatomic particles; A quantum number that determines the electromagnetic interactions of some subatomic particles; Memorize relative charge values and amu. the nuclei of all atoms contain subatomic particles called protons close proton subatomic particle with a positive charge and a. What Are The Electrical Charges Of Subatomic Particles.
From www.sliderbase.com
The Atom Presentation Chemistry What Are The Electrical Charges Of Subatomic Particles Discovered by ernest rutherford in 1919. Memorize relative charge values and amu. For example, electrons have negative charge and. Reside in the nucleus of an atom. Approximately 1 atomic mass unit (amu). A quantum number that determines the electromagnetic interactions of some subatomic particles; know the basics of the experiments involving the discoveries of the three subatomic particles; . What Are The Electrical Charges Of Subatomic Particles.
From www.alamy.com
Illustration of a neutron, a subatomic particle with no net electric What Are The Electrical Charges Of Subatomic Particles Discovered by james chadwick in 1932. Memorize relative charge values and amu. Approximately 1 atomic mass unit (amu). For example, electrons have negative charge and. Reside in the nucleus of an atom. A quantum number that determines the electromagnetic interactions of some subatomic particles; A quantum number that determines the electromagnetic interactions of some subatomic particles; Discovered by ernest rutherford. What Are The Electrical Charges Of Subatomic Particles.